Pattern formation phenomena
 Ordered structures and patterns can be spontaneously formed from initial homogeneous systems. Such pattern formation phenomena appear in both living and non-living systems. Beautiful body surface patterns of animals are also realized by physicochemical mechanism similar to ordered pattern observed in non-living systems, not by genetic code. In our lab., we enjoy to find new types of pattern formation phenomena experimentally, and try to uncover the mechanism for them.
Ordered structures and patterns can be spontaneously formed from initial homogeneous systems. Such pattern formation phenomena appear in both living and non-living systems. Beautiful body surface patterns of animals are also realized by physicochemical mechanism similar to ordered pattern observed in non-living systems, not by genetic code. In our lab., we enjoy to find new types of pattern formation phenomena experimentally, and try to uncover the mechanism for them.
The Belousov-Zhabotinsky (BZ) reaction
 The Belousov-Zhabotinsky (BZ) reaction, typical chemical oscillatory reaction, occurs in an aqueous solution of bromate ion, acid, malonic acid, and metal catalyst. This is nonlinear chemical reaction and can show ordered pattern formation. Beautiful target pattern or spiral pattern can be observed in this reaction. Those phenomena have been well explain using mathematical model.
The Belousov-Zhabotinsky (BZ) reaction, typical chemical oscillatory reaction, occurs in an aqueous solution of bromate ion, acid, malonic acid, and metal catalyst. This is nonlinear chemical reaction and can show ordered pattern formation. Beautiful target pattern or spiral pattern can be observed in this reaction. Those phenomena have been well explain using mathematical model.
【Related Videos】
“Target pattern in the BZ reaction:”
A small blue dot is generated in the initial homogeneous red solution, and it slowly expands. This oxidation periodically occurs at the same position. Thus, beautiful ordered target pattern is spontaneously formed after several minutes.
【Related Articles】
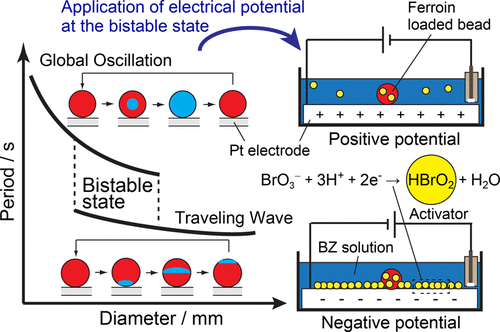
- Chemical Wave Propagation in the Belousov?Zhabotinsky Reaction Controlled by Electrical Potential
- JPC A 123, 4853 (2019).
- Link to Paper
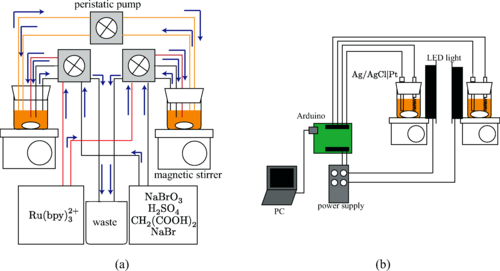
- Competition between global feedback and diffusion in coupled Belousov-Zhabotinsky oscillators
- Phys. Rev. E 99, 012208 (2019).
- Link to Paper
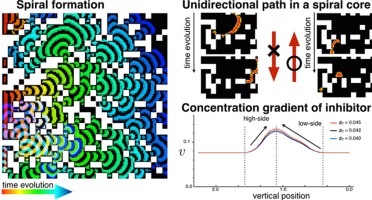
- "Spontaneous Formation of Unidirectional Path"
- Chem. Phys. Lett. 616-617, 248-253 (2014).
- Link to Paper

- "Density Wave Propagation of a Wave Train in a Closed Excitable Medium"
- Phys. Rev. E 84, 046203 (2011).
- Link to Paper
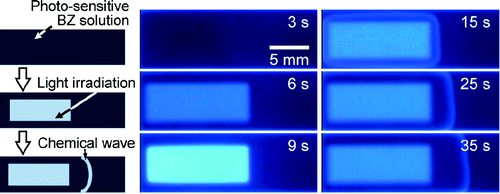
- "Photoexcitaed Chemical Wave in the Ruthenium-Catalyzed Belousov-Zhabotinsky Reaction"
- JPC A 115, 7406 (2011).
- Link to Paper
pattern appeared on a precipitate plate of aluminum hydroxide
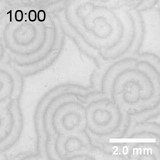 Aluminum ion react to hydroxide ion and produce precipitate, and further adding hydroxide ion dissolute the precipitate. That’s why if hydroxide ion solution puts on a gel including aluminum ion, thin band shaped precipitate is generated and it slowly shifts toward the bottom due to the diffusion of hydroxide ion. On this precipitate band, defects shape spiral pattern and it spontaneously moves. The mechanism of this interesting behavior is still under investigation.
Aluminum ion react to hydroxide ion and produce precipitate, and further adding hydroxide ion dissolute the precipitate. That’s why if hydroxide ion solution puts on a gel including aluminum ion, thin band shaped precipitate is generated and it slowly shifts toward the bottom due to the diffusion of hydroxide ion. On this precipitate band, defects shape spiral pattern and it spontaneously moves. The mechanism of this interesting behavior is still under investigation.
【Related Videos】
“Spiral pattern generated on a precipitation band of aluminum hydroxide:”
Aluminum ion react to hydroxide ion and produce precipitate, and further adding hydroxide ion dissolute the precipitate. That’s why if hydroxide ion solution puts on a gel including aluminum ion, thin band shaped precipitate is generated. On this band, spiral pattern is generated.
【Related Articles】
None