Welcome to Nagashima Group homepage
Kazushige Nagashima

Associate Professor, Department of Physics, Meiji University
Education
1993 B.S., Physics, Meiji University, Kanagawa, Japan
1997 Ph.D., Environmental Earth Science, Hokkaido University, Sapporo, Japan
Ph.D Theses: Experimental studies on pattern formation of ice crystals during directional growth
Experience
1997-2001 Post doctoral researcher: National Institute for Resources and Environment, Japan
2001-2005 Lecturer of Physics, Meiji University, Kanagawa, Japan
2005-2012 Associate Professor
2001- Visiting researcher of AIST
2013- Professor
Contact Information
Kazushige Nagashima
Address: 1-1-1 Higashimita, Tama-ku, Kawasaki, 214-8571, Japan
E-mail: knaga(at)isc.meiji.ac.jp
*replace (at) to @
Phone/FAX: +81-44-934-7269
Current research projects
Formation of clathrate hydrates in porous media
Formation mechanism of clathrate hydrates
Pattern formation of crystals in Storm Glass
Compositional convection induced by ice growth in saline solution
Research Description
Formation of clathrate hydrates in porous media
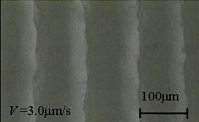 Clathrate hydrates are crystalline inclusion compounds, which are capable of hosting small molecules inside the cages of a hydrogen-bonded water framework. Methane hydrates found globally in sediments under the ocean floor are of significant interest both as a possible cause of global climate change and as a potential energy resource. Cores recovered from the ocean floor have been reported to have a variety of patterns (textures) and sizes of hydrates in the sediments, and were classified into four categories by Malone: disseminated (fine hydrates in the pore space of sediments), nodular (hydrates up to 5 cm in diameter), layered (parallel hydrate layers), and massive (as thick as 3–4 m and containing 95% hydrates and less than 5% sediments). We have recently demonstrated (J. Physical Chemistry B 2008) that layered patterns of pure hydrates (Tetrahydrofuran hydrates used as a model) were formed in the mixture as the growth of hydrate pushed the silica beads (frost heaving of hydrates). The pattern formation of layered hydrates was systematically studied as a function of the growth rate, applied temperature gradient, and weight ratio of the solution and the silica beads (the solution content in the mixture). The ultimate goal of our research is to advance our understanding of the formation kinetics and formation time scale of natural methane hydrates in oceanic sediments.
Clathrate hydrates are crystalline inclusion compounds, which are capable of hosting small molecules inside the cages of a hydrogen-bonded water framework. Methane hydrates found globally in sediments under the ocean floor are of significant interest both as a possible cause of global climate change and as a potential energy resource. Cores recovered from the ocean floor have been reported to have a variety of patterns (textures) and sizes of hydrates in the sediments, and were classified into four categories by Malone: disseminated (fine hydrates in the pore space of sediments), nodular (hydrates up to 5 cm in diameter), layered (parallel hydrate layers), and massive (as thick as 3–4 m and containing 95% hydrates and less than 5% sediments). We have recently demonstrated (J. Physical Chemistry B 2008) that layered patterns of pure hydrates (Tetrahydrofuran hydrates used as a model) were formed in the mixture as the growth of hydrate pushed the silica beads (frost heaving of hydrates). The pattern formation of layered hydrates was systematically studied as a function of the growth rate, applied temperature gradient, and weight ratio of the solution and the silica beads (the solution content in the mixture). The ultimate goal of our research is to advance our understanding of the formation kinetics and formation time scale of natural methane hydrates in oceanic sediments.
Pattern formation of crystals in Storm Glass
“Storm glass” is a sealed glass tube containing a camphor-ethanol solution with aqueous NH4Cl and KNO3 solution. In 19th century England, the pattern and quantity of the crystals formed were observed and interpreted as a weather forecasting tool. We demonstrated that (i) typical crystal patterns that appear in storm glass were obtained during the directional growth by controlling the growth rates (cooling rate). In addition, minor components (potassium nitrate and ammonium chloride) and water in the solution controlled the nucleation of the camphor crystals at higher values of the growth rates. (ii) XRD showed that the crystals in the storm glass were camphor. (iii) The history of the periodic temperature variation affected the pattern and the quantity of the crystals. In case of the daily variations in temperature, the appearance of the crystals was affected by the temperature history over several days.
 The ultimate goal of our research is to advance our understanding of the effect of daily variations of temperature on numerous appearances of crystals, the diffusion processes of camphor, potassium nitrate, and ammonium chloride caused by both growth and melting of crystals in water-ethanol solution, and the interactions between molecules. Furthermore, the storm glass is vertically arranged; therefore, the effect of gravity on the convection in the solution is also important. Detailed investigations of this nature are currently underway.
The ultimate goal of our research is to advance our understanding of the effect of daily variations of temperature on numerous appearances of crystals, the diffusion processes of camphor, potassium nitrate, and ammonium chloride caused by both growth and melting of crystals in water-ethanol solution, and the interactions between molecules. Furthermore, the storm glass is vertically arranged; therefore, the effect of gravity on the convection in the solution is also important. Detailed investigations of this nature are currently underway.
Selected Publications
Michihiro Muraoka, Kazushige Nagashima「Pattern variety of tetrahydrofuran clathrate hydrates formed in porous media」 J. Physical Chemistry C, vol.116, No.44(2012)pp.23342–23350
T. Suzuki, M. Muraoka, K. Nagashima, Foreign particle behavior at the growth interface of tetrahydrofuran clathrate hydrates, J. Crystal Growth, Vol. 318 (2011) p. 131-134,.
Y.Sabase, K.Nagashima, Growth mode transition of tetrahydrofuran clathrate hydrates in the guest/host concentration boundary layer, J. Physical Chemistry B,113(2009)15304-15311.
K. Nagashima, T. Suzuki, M. Nagamoto, T. Shimizu, Formation of periodic layered pattern of tetrahydrofuran clathrate hydrates in porous media,
J. Physical Chemistry B, 112(2008)9876-9882.
Yasuko Tanaka, Koichi Hagano, Tomoyasu Kuno, Kazushige Nagashima, Pattern formation of crystals in Storm glass,
J. Crystal Growth, 310(2008)2668-2672.
K. Nagashima, S. Orihashi, Y. Yamamoto, M. Takahashi, Encapsulation of Saline Solution by Tetrahydrofuran Clathrate Hydrates and Inclusion Migration by Recrystallization,
J. Physical Chemistry B, 109(2005)10147-10153.
Kazushige Nagashima, Yoshinori Furukawa, Effects of gravity on directional growth and melting of ice crystals in solution,
Canadian J. Physics, 81 (2003) 99-105.
Kazushige Nagashima, Yoshinori Furukawa, Interferometric observation of the effects of gravity on the horizontal growth of ice crystals in a thin growth cell,
Physica D, 147(2000)177-186.
Kazushige Nagashima, Yoshinori Furukawa, Time development of a solute diffusion field and morphological instability on a planar interface in the directional growth of ice crystals,
J. Crystal Growth, 209(2000)167-174.
Kazushige Nagashima, Yoshinori Furukawa, Solute distribution in front of an ice-water interface during directional growth of ice crystals and its relationship to interfacial patterns,
J. Physical Chemistry B, 101 (1997)6174-6176.
Kazushige Nagashima, Yoshinori Furukawa, Nonequilibrium effect of anisotropic interface kinetics on the directional growth of ice crystals,
J. Crystal Growth, 171(1997)577-585.
