Nagashima, Kazushige Professor
 |
|
Research Interests
Pattern formation of crystals has been investigated using many kinds of materials under a wide range of growth conditions because of the physico-chemical interest in nonlinear and nonequilibrium phenomena. We have particular interest in the pattern formation mechanism of crystals composed of water molecules such as ice and clathrate hydrates. The growth of these crystals has received much attention in various research fields: earth science, cryobiology, development of natural resources, and so on. We also study the behavior of camphor crystals in storm glass.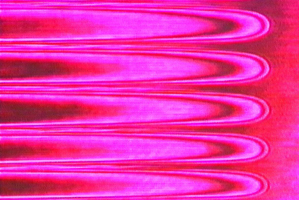 |
| Fig. 1 Cellular pattern of ice crystals and contour lines of crystal thickness shown by interference fringes |
Ice crystals in saline solution form cellular and dendrite patterns during directional growth (Fig. 1). The diffusion of salt (impurity) in solution has a critical effect on the pattern and sizes of crystals. Our advantage lies in interferometric technique to observe the time development of diffusion field of salt as well as the growth pattern in order to clarify the pattern formation mechanism.
(ii) Pattern formation of THF hydrates in porous media ~An attempt to clarify pattern formation mechanism of methane hydrates in oceanic sediments~
Methane hydrates in oceanic sediments have a variety of patterns (textures) and sizes. We succeeded in forming all patterns of hydrates in the mixture of THF water solution and silica beads used as a model. Our goal is to clarify the pattern formation mechanism by combining the model of frost heave and the model of pattern formation of crystal, and predict the formation conditions and time scale of natural hydrates.
(iii) Crystal behavior in storm glass
Storm glass is a sealed glass tube containing camphor crystals in solution. The pattern and quantity of the crystals were observed and interpreted as a weather forecasting tool mainly in 19th century England. We succeeded in forming typical crystal patterns that appear in storm glass. We found that the pattern and quantity of the crystals were affected by the temperature history over several days. Our ultimate goal is to clarify the formation mechanism of various appearances of crystals under day-by-day variation of temperature.
Education
| 1993 | B.S., Physics, Meiji University, Japan |
| 1997 | Ph.D., Environmental Earth Science, Hokkaido University, Japan |
| Ph.D. Theses: Experimental studies on pattern formation of ice crystals during directional growth | |
Professional Experience
| 1997–2001 | Post doctoral researcher: National Institute for Resources and Environment, Japan |
| 2001–2005 | Lecturer, Department of Physics, Meiji University, Japan |
| 2005–present | Associate Professor, Department of Physics, Meiji University, Japan |
| 2001–present | Visiting researcher of AIST, Japan |
Selected Publications
- M. Muraoka, K. Nagashima, “Pattern variety of tetrahydrofuran clathrate hydrates formed in porous media”, J. Physical Chemistry C, 116, No.44, 23342–23350, (2012).
- T. Suzuki, M. Muraoka, K. Nagashima, “Foreign particle behavior at the growth interface of tetrahydrofuran clathrate hydrates”, J. Crystal Growth, 318, No.1 131-134, (2011).
- Y. Sabase, K. Nagashima, “Growth mode transition of tetrahydrofuran clathrate hydrates in the guest/host concentration boundary layer”, J. Physical Chemistry B, 113, No. 46, 15304-15311, (2009).
- K. Nagashima, T. Suzuki, M. Nagamoto, T. Shimizu, “Formation of periodic layered pattern of tetrahydrofuran clathrate hydrates in porous media”, J. Physical Chemistry B, 112, No.32, 9876-9882, (2008).
- Y. Tanaka, K. Hagano, T. Kuno, K. Nagashima, “Pattern formation of crystals in Storm glass”, J. Crystal Growth, 310, No.10, 2668-2672, (2008).
- K. Nagashima, S. Orihashi, Y. Yamamoto, M. Takahashi, “Encapsulation of Saline Solution by Tetrahydrofuran Clathrate Hydrates and Inclusion Migration by Recrystallization”, J. Physical Chemistry B, 109, 10147-10153, (2005).
- K. Nagashima, Y. Furukawa, “Interferometric observation of the effects of gravity on the horizontal growth of ice crystals in a thin growth cell”, Physica D, 147, 177-186, (2000).








